Today, the U.S. Food and Drug Administration took an important step to increase racial and ethnic diversity in clinical trials by releasing new draft guidance to the industry. The guidance comes as a group of legislators seeks to compel pharmaceutical companies to do more to encourage diverse clinical enrollment for the drugs they sponsor. Current legislative bills to tackle clinical trial diversity include:
- Cures 2.0 (H.R. 6000)
- The DIVERSE Trials Act (H.R. 5030)
- The Equity in Neuroscience and Alzheimer’s Clinical Trials Act of 2021 (H.R. 3085)
The pharmaceutical industry trade group has exerted considerable effort to get legislators to back off specific mandates aimed at improving the diversity of clinical trial participants. The new FDA guidance stops short of mandates but requires pharmaceutical companies to develop specific plans to ensure the representative enrollment of certain populations in their pivotal studies.
Specifically, the guidance informs pharmaceutical sponsors of clinical trials to:
- Describe in detail the operational measures that will be implemented to enroll and retain underrepresented racial and ethnic participants in the planned trial(s) or studies, and the planned use of data to characterize safety, efficacy, and optimal dosage in these participants, when applicable.
- Describe specific trial enrollment and retention strategies, including but not limited to site location and access, sustained community engagement, and reducing burdens due to trial/study design/conduct.
- Describe metrics to ensure that diverse participant enrollment goals are achieved and specify actions to be implemented during the conduct of the trial(s) or studies if planned enrollment goals are not met.
Over the past two years, Acclinate has worked or consulted with numerous pharmaceutical companies in their efforts to achieve adequate racial and ethnic diversity in their clinical trials. Specifically, we’ve had in-depth discussions with twenty-five drug sponsors, covering the spectrum from emerging biopharma to international pharmaceutical companies with household names. What emerged from those discussions was a taxonomy for categorizing each company’s posture to the imminent industry and regulatory changes based on their strategic orientation and proactiveness.
Strategic orientation can be defined as identifying a current state and intended future state, complete with the main goals and objectives, and then building the corresponding vision, mission, values, and procedural plan to achieve them. Proactiveness can be defined as anticipating challenges, seeking new solutions, and taking intervening action. These are often self-initiated behaviors intended to solve a problem before they occur or become worse.
A 2x2 matrix with four distinct quadrants emerged when we combined the level of strategic orientation and the level of proactiveness exhibited by the twenty-five pharmaceutical companies we've worked or consulted with.
Figure: 2x2 Matrix of 25 Pharma Companies
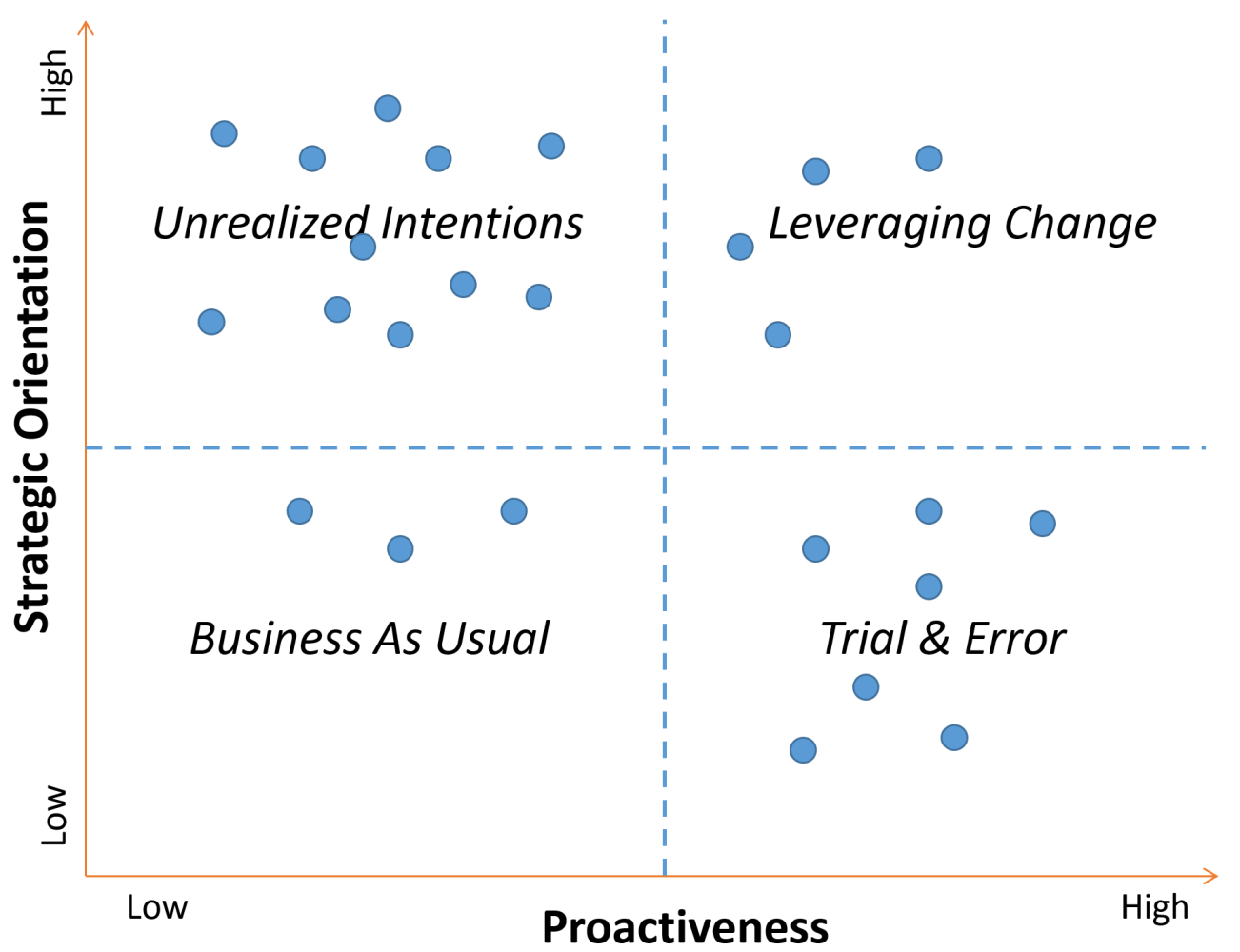
Business as Usual
Pharmaceutical companies in this quadrant have low strategic orientation and low proactiveness. One might refer to this as the proverbial “head in the sand” approach of refusing to acknowledge an imminent external change, even when not responding to the change may be existential. The good news is that only 10% of the pharmaceutical companies that we’ve interacted with reside in this quadrant. Recent examples of the FDA not granting drug approval for trials without adequate racial and ethnic representation is a wake-up call for pharmaceutical companies to move from this posture quickly.
Unrealized Intentions
Pharmaceutical companies in this quadrant have high strategic orientation but low proactiveness. Nearly half the pharma companies fall within this quadrant. These companies suffer from “analysis paralysis” and often have meetings to plan for their meetings and working groups within subcommittees of the larger group. You might hear a DEI lead from a pharmaceutical company in this quadrant say, “This year we will develop the plan, next year we will get the plan approved, and then in year three, establish benchmarks against the plan.” Companies in this quadrant may be relatively safe for the short term based on the new FDA guidance. However, they will significantly erode their competitive advantage and lead in the marketplace related to clinical trial diversity.
Trial & Error
Pharmaceutical companies in this quadrant have low strategic orientation but high proactiveness. Approximately 30% of pharmaceutical companies fall within this quadrant. They effectively recognize there is no silver bullet solution to clinical trial diversity, but they overcompensate by using the equivalent of a low-powered shotgun. With just a little planning, they are off to the races on several piecemeal “initiatives” – new connections with patient advocacy groups, supporting efforts to diversify the industry’s principal investigators, interviewing decentralized clinical trial providers, and spinning up new sites in majority-minority metro areas. These pharma companies will see some improvement in their clinical trial diversity, especially compared to companies residing in the Unrealized Intentions quadrant. The incremental gains may come with significant investment but a nominal ROI.
Leveraging Change
Pharmaceutical companies in this quadrant have high strategic orientation and high proactiveness. Approximately 10% of the pharmaceutical companies we’ve interacted with fall within this quadrant. These companies have adopted a type of strategic agility that allows them to accurately anticipate future consequences and trends, recognize strategic opportunities created by the change, and implement innovative strategies. They often utilize pilot programs to run planning, implementing, learning, and iterating cycles. Pharma companies in this quadrant objectively assess their clinical trial diversity against a standardized metric and produce an upward trend line from the process. They also see how the modifications meant to increase diversity can positively drive key performance indicators like patient accrual and retention. Pharmaceutical companies in this quadrant will utilize the recent FDA guidance to establish themselves as market leaders further.
"A Change Gonna Come"
The pharmaceutical industry has been characterized as slow to change. However, history tells us that the industry can quickly adapt when faced with regulator pressures and mandates designed to provide better accountability and outcomes (e.g., IRB and HIPAA). The FDA first issued its draft guidance on enhancing the diversity of clinical trial populations in 2019. Today's updated guidance aimed at achieving adequate diversity and representation of clinical trial participants is long overdue. As the songwriter, entrepreneur, and activist Sam Cooke said, “It's been a long time coming, but I know a change gonna come.” Now is the time for pharmaceutical companies to show the strategic agility to ensure health equity through diverse and inclusive clinical research.









.webp)




